

OLSSON'S IS CLOSED
Thank you to all our loyal customers who supported us for 36 years
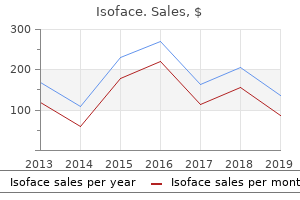
"Generic 40mg isoface fast delivery, skin care vitamins".
By: I. Malir, M.A., M.D.
Clinical Director, Medical University of South Carolina College of Medicine
Central nervous system and limb anomalies in case-reports of first trimester statin exposure skin care giant cheap isoface express. Mechanistic and epidemiologic issues in the evaluation of adverse birth outcomes following gestational publicity to statins retinol 05 acne generic isoface 5mg mastercard. The drug triggered embryofetal toxicity in two animal species at systemic exposures lower than the human exposure acne 9 year old buy isoface from india. If a pregnant girl is exposed to this drug skin care 1 month before wedding purchase generic isoface line, she ought to be informed of the absence of human pregnancy experience and the potential for embryofetal danger. It is indicated for adjunctive therapy of seizures associated with Lennox-Gastaut syndrome in youngsters 4 years of age and adults. Only 34% of the drug is bound to plasma proteins, largely to albumin, and the plasma half-life is about 610 hours (1). In rabbits, daily oral doses throughout organogenesis resulting in exposures that have been >0. In prenatal and postnatal research with rats, daily oral doses given from implantation through weaning ensuing plasma exposures that were <0. Long-term exposures to rufinamide in mice and rats had been carcinogenic, but the drug was not mutagenic or clastogenic in a number of assays. The low molecular weight (about 238), lipophilic nature, and lengthy plasma half-life counsel that exposure of the embryofetus will occur. The low molecular weight (about 238), lipophilic nature, and long plasma half-life suggest that the drug might be excreted into breast milk, however the in depth metabolism ought to restrict the quantity. If mother chooses to nurse her toddler while taking rufinamide, the toddler ought to be carefully observed for adjustments in habits and different indicators of toxicity. Somnolence, vomiting, and headache have been the three most frequent opposed reactions observed in pediatric sufferers handled with the drug (1). The animal knowledge advised danger (reduced fetal weights and late resorptions in two species) however these results occurred with doses that have been maternally poisonous. However, decrease nonmaternal toxic doses in rats had been related to postimplantation losses. If the drug is indicated in a pregnant lady, she must be knowledgeable of the potential danger to her embryofetus. It is indicated for the remedy of patients with intermediate- or high-risk myelofibrosis, including main myelofibrosis, postpolycythemia vera myelofibrosis, and postessential thrombocythemia myelofibrosis. At these doses, lowered fetal weights had been noted in rats and rabbits, and elevated late resorptions in rabbits, however maternal toxicity occurred in each species. Ruxolitinib was not carcinogenic in long-term studies in mice and rats, and was not mutagenic or clastogenic in a number of assays. The molecular weight of the parent drug (about 404) suggests that the drug will cross, but the excessive plasma protein binding and comparatively short mean elimination half-lives should restrict the exposure. The molecular weight of the parent drug (about 404) suggests that the drug, and presumably its two energetic metabolites, shall be excreted into breast milk, but the excessive (97%) plasma protein binding and comparatively quick mean elimination half-lives (3 hours for the mother or father drug and 5. However, thrombocytopenia and anemia occurred in >20% of sufferers treated with the drug and >10% experienced bruising, dizziness, and headache (1). Thus, if the drug is given during breastfeeding, a nursing toddler should be monitored for these opposed effects. The Calorie Control Council believes that the agent may be safely utilized by pregnant women (1). However, others really helpful avoidance of saccharin or, no less than, cautious use of it in pregnancy (24). Saccharin, a derivative of naphthalene, is absorbed slowly after oral ingestion and is quickly and fully excreted, as the unmetabolized compound, by the kidneys. Although a considerable amount of medical analysis has been generated regarding saccharin, little or no of this information pertains to its use by pregnant women or to its impact on the fetus (2,3). Fetal ranges were still current 5 hours after the top of the infusion and a pair of hours after maternal concentrations had been undetectable. A research, printed in 1986, documented that saccharin additionally crosses the placenta to the human fetus (6). Six diabetic girls, consuming 25100 mg/day of saccharin by historical past, had been delivered at 3642 weeks.
Most reviews have found no adverse results on delivery weight acne vitamin deficiency generic 40 mg isoface fast delivery, head circumference acne 7 days past ovulation cheap isoface 20 mg with amex, Apgar scores acne hat discount 20mg isoface free shipping, or blood glucose control after in utero exposure to labetalol (913) skin care natural buy generic isoface 5 mg on-line. One case of neonatal hypoglycemia has been talked about, however the mother was also taking a thiazide diuretic (2). Offspring of moms treated with labetalol had a significantly larger birth weight than infants of atenolol-treated mothers, 3280 vs. Fetal coronary heart rate is seemingly unaffected by labetalol remedy of hypertensive pregnant ladies. In considered one of these infants, bradycardia was marked (<100 beats/minute) and protracted (17). In a examine analyzing the consequences of labetalol publicity on term (37 weeks) newborns, delicate transient hypotension, which resolved inside 24 hours, was noticed in 11 infants compared with 11 matched controls (18). Maternal dosage varied from a hundred to 300 mg three instances daily with the final dose given inside 12 hours of start. The mean systolic blood pressures at 2 hours of age in exposed and nonexposed infants were fifty eight. Several investigations have proven an absence of impact of labetalol remedy on uterine contractions (13,sixteen,1921). One study did report a higher incidence of spontaneous labor in labetalol-treated mothers (6 of 10) than in an identical group treated with methyldopa (2 of 9) (22). In another report, 3 of 31 patients handled with labetalol experienced spontaneous labor, one of whom delivered prematurely (23). The authors attributed the uterine activity to the drug as a result of no other causes were discovered. The lack of effect on blood move was in all probability caused by lowered peripheral resistance. Labetalol apparently reduces the incidence of hyaline membrane disease in premature infants by growing the production of pulmonary surfactant (1,2,four,sixteen,26). The mechanism for this impact could additionally be mediated through 2adrenoceptor agonist activity that the drug partially possesses (1,2,four,16,26). Follow-up studies have been completed at 6 months of age on 10 infants exposed in utero to labetalol (27). In addition, no ocular toxicity was noticed in newborns, although labetalol has an affinity for ocular melanin (1,2,26). The experience with labetalol in pregnancy was briefly reviewed in a 1988 article (31). In 24 lactating women at three days postpartum, administration of 330800 mg/day produced a imply milk stage of 33 ng/mL. Three ladies at 69 days postpartum consumed day by day doses of labetalol of 600, 600, and 1200 mg and produced peak milk concentrations of the drug of 129, 223, and 662 ng/mL, respectively (6). Peak concentrations of labetalol in the milk occurred between 2 and 3 hours after a dose. Measurable plasma concentrations of labetalol have been found in only one infant: 18 ng/mL at 4 hours and 21 ng/mL at 8 hours. Although no adverse results have been reported, nursing infants must be carefully observed for bradycardia, hypotension, and other symptoms of /-blockade. The American Academy of Pediatrics classifies labetalol as appropriate with breastfeeding (33). Effect of labetalol on hypertension and the reninangiotensinaldosterone and adrenergic methods in being pregnant. Acute effects of labetalol on maternal metabolism and uteroplacental circulation in hypertension of being pregnant. Comparison of the alpha and beta blocking drug, labetalol, and methyl dopa in the therapy of moderate and severe pregnancy-induced hypertension. The fetal end result in a randomized trial of labetalol versus placebo in pregnancy-induced hypertension. A comparison of labetalol plus hospitalization versus hospitalization alone within the management of preeclampsia remote from term. A comparison of labetalol with different antihypertensive drugs in the remedy of hypertensive illness of pregnancy. Intravenous labetalol and intravenous dihydralazine in severe hypertension in pregnancy. A controlled trial of the remedy of hypertension in pregnancy: labetalol compared with methyldopa.
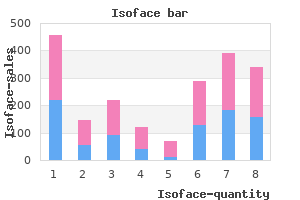
Buy isoface 40 mg with visa. A medical estheticians skin care routine How to have the best skin 2017.
Finally acne zones and meaning buy discount isoface 30mg online, 25 retrospective reports of infants with anomalies acne reviews safe isoface 20mg, who had been uncovered in utero to fluoroquinolones skin care heaven coupon order isoface 5mg with amex, have been analyzed skincare for 40 year old woman buy generic isoface on line, however no specific patterns of major congenital malformations have been detected. The authors of the above examine concluded that pregnancy exposure to quinolones was not an indication for termination, however that this class of antibacterial agents should nonetheless be thought of contraindicated in pregnant ladies. No stories describing the usage of lomefloxacin in human lactation have been situated. Other quinolones are excreted into milk and, due to its relatively low molecular weight (about 388), the passage of lomefloxacin into milk should be anticipated. The American Academy of Pediatrics classifies ciprofloxacin and ofloxacin as compatible with breastfeeding (see Ciprofloxacin and Ofloxacin). The producer classifies the drug as contraindicated due to the toxicity noticed in three animal species. The defects included umbilical hernia, gastroschisis, imperforate anus, alterations in heart form and dimension, limb malrotations, skeletal malformations of the tail, and delayed ossification of cranial, vertebral and pelvic bones. Defects included umbilical hernia, medially rotated or brief limbs, absent or fused digits on paws, cleft palate, open eye lids, low-set ears, and kinked tail (1). In a 2-year dietary carcinogenicity examine in mice, lomitapide triggered significant increases of liver adenomas and small gut carcinomas in males and combined adenomas and carcinomas in females. In 2-year research in rats, there have been no statistically significant drug-related will increase in tumor incidences. The molecular weight (about 790) and high plasma protein binding counsel that publicity of the embryofetus will be limited, however the long terminal half-life may permit the drug to cross. However, if a mom receiving the drug chooses to breastfeed, the infant must be monitored for the most typical (incidence 28%) opposed effect seen in adults. These effects embody diarrhea, nausea, vomiting, dyspepsia, and belly ache (1). No revealed reviews linking the utilization of loperamide with congenital defects have been located. Reproduction studies with rats and rabbits at doses as a lot as 30 times the human dose have revealed no proof of impaired fertility, teratogenicity, or other fetal hurt (1). In a surveillance study of Michigan Medicaid recipients involving 229,one hundred and one accomplished pregnancies carried out between 1985 and 1992, 108 newborns had been exposed to loperamide in the course of the 1st trimester (F. In a 1999 summary, the being pregnant outcomes of 89 women uncovered to loperamide within the 1st trimester have been in contrast with matched controls (2). There have been no important differences between the groups by means of main and minor malformations, spontaneous abortions, elective abortions, preterm supply, and delivery weights. However, in 21 mothers who took loperamide throughout gestation, delivery weights tended to be lower (200 g) (ns) (2). Simultaneous plasma and milk samples had been collected 12 hours after the first dose, and 6 and 24 hours after the second dose. Small quantities of loperamide oxide have been measured in a few of the plasma samples, but the imply loperamide oxide milk concentrations had been <0. The American Academy of Pediatrics classifies loperamide as appropriate with breastfeeding (4). The animal knowledge are suggestive of average threat as a end result of the publicity for lopinavir was less than the human therapeutic exposure. The agent is simply out there in a fixed mixture (200 mg lopinavir/50 mg ritonavir per capsule; eighty mg lopinavir/20 mg ritonavir per mL oral solution). The plasma ranges of ritonavir are very low; subsequently, the antiviral exercise of the mixture is due to lopinavir. Plasma protein binding of lopinavir is high (98%99%), primarily by 1-acid glycoprotein, but some is sure to albumin. The average half-life of lopinavir over a 12-hour dosing interval is 56 hours (1). Developmental toxicity (decreased pup survival) additionally was observed in a perinatal and postnatal rat research at doses producing exposures equal to or larger than about zero. Consistent with the molecular weight (about 629) and lipid solubility, lopinavir crosses the human placenta. A 2006 research using the ex vivo human cotyledon perfusion model found that placental transfer of lopinavir (combined with ritonavir) was appropriate with passive diffusion, even within the presence of physiologic concentrations of human albumin (2). The quantity coming into the fetal compartment was nicely above the 50% inhibitory concentration (2). The Antiretroviral Pregnancy Registry reported, for January 1989 by way of July 2009, potential data (reported before the outcomes were known) involving 4702 stay births that had been uncovered through the 1st trimester to one or more antiretroviral brokers (5).

His improvement during the first yr was regular acne 17 year old male buy isoface 5 mg cheap, as was his present serum ceruloplasmin focus skin care talk cheap isoface 40 mg free shipping, however his liver enzymes remained elevated acne 35 weeks pregnant purchase isoface 30 mg free shipping, presumably due to copper accumulation in the fetal liver (21) skin care equipment suppliers cheap isoface 30mg with amex. Several conflicting recommendations have appeared in the literature concerning using penicillamine throughout being pregnant. The authors of 1 evaluate consider the drug ought to be prevented throughout being pregnant (22). However, a 2000 evaluation said that no antagonistic effects in nursing infants have been reported by moms taking penicillamine, despite the very fact that one examine did discover lower amounts of zinc and copper in milk (20). Dissolution of cystine stones during dpenicillamine therapy of a pregnant patient with cystinuria. Congenital connective-tissue defect most likely as a outcome of d-penicillamine remedy in being pregnant. Neonatal abnormalities associated with d-penicillamine treatment throughout being pregnant. Presented at the 13th Annual Birth Defects Conference, June 1980, March of Dimes and University of California, San Diego. Reproduction studies in mice, rats, and rabbits revealed no proof of impaired fertility or fetal hurt (1). Therapeutic levels are reached in both sites apart from the amniotic fluid during the 1st trimester (6). The early use of penicillin G was linked to increased uterine activity and abortion (711). An anaphylactic reaction in a pregnant patient reportedly led to the death of her fetus in utero (12). Only one reference has linked the use of penicillin G with congenital abnormalities (13). An examination of hospital information indicated that in three of 4 instances the administration of penicillin G had been followed by the start of a malformed child. A retrospective review of further sufferers uncovered to antibiotics within the 1st trimester indicated a rise in congenital defects. In a managed examine, 110 sufferers obtained one to three antibiotics in the course of the 1st trimester for a complete of 589 weeks (16). The incidence of start defects was no completely different from that in a nontreated control group. Although no antagonistic effects were reported, three potential issues exist for the nursing infant: modification of bowel flora, direct results on the toddler. Its usefulness, limitations, diffusion and detection, with analysis of a hundred and fifty cases by which it was employed. Transplacental passage of azidocillin, ampicillin and penicillin G throughout early and late being pregnant. Intrauterine fetal death as a outcome of anaphylactic response to penicillin in a pregnant girl. On the potential teratogenicity of antibiotic medication administered during pregnancy-a prospective examine. Reproduction research in mice, rats, and rabbits have revealed no evidence of impaired fertility or fetal harm (1). Penicillin concentrations had been measured in maternal serum, maternal cerebrospinal fluid, wire serum, and amniotic fluid. Because of the wide selection of concentrations measured, the authors concluded that the altered pharmacokinetics occurring right now may adversely affect the efficacy of the drug to stop congenital syphilis. Penicillin levels following the administration of benzathine penicillin G in pregnancy. Reproduction research in mice, rats, and rabbits have revealed no proof of impaired fertility or fetal harm (2) (see additionally Penicillin G). In a surveillance research of Michigan Medicaid recipients involving 229,101 completed pregnancies carried out between 1985 and 1992, 4597 newborns had been exposed to penicillin V in the course of the 1st trimester (F. Specific data were obtainable for six defect categories, including (observed/expected) 46/56 cardiovascular defects, 5/7 oral clefts, 3/2 spina bifida, 17/13 polydactyly, 7/8 limb discount defects, and 8/11 hypospadias. Urinary estriol was formerly used to assess the situation of the fetoplacental unit, with depressed levels being related to fetal misery. The pharmacokinetics of penicillin V during the 2nd and 3rd trimesters have been reported (3). Although no developmental toxicity has been observed with this agent, some sources think about it contraindicated in pregnancy.